Exactly What is a Polymer?
Are Polymers Chemicals? How Do Polymers Create Energy?
As defined by Merriam-Webster.com, a polymer is “a chemical compound or mixture of compounds formed by polymerization and consisting essentially of repeating structural units”. Again Merriam-Webster, polymerization is “a chemical reaction in which two or more molecules combine to form larger molecules that contain repeating structural units”. Thoughtco.com defines a polymer as “a chemical compound with molecules bonded together in long, repeating chains”. A couple final definitions, and I think we get the picture. A molecule is “the smallest particle of a substance that retains all the properties of the substance and is composed of one or more atoms”, so says Merriam-Webster. And we all can remember from elementary school that the atom is the smallest particle of an element.
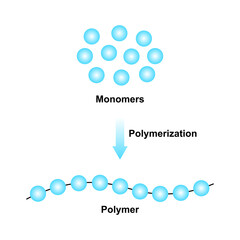
Atom > molecule > monomer > polymerization > Polymers! So now, what do we do with these? How do these create energy?

It’s easy to assume that all this is chemistry, in some form. Some clarification is needed. While many polymers are man-made (synthetic), others are made by mother nature, and these are referred to as natural polymers. Rubber, shellac, cellulose are examples of natural polymers. Rubber with its elastic qualities comes from trees. Shellac is produced by the lac bug and used as paint primer, sealant, and varnish. The most common natural polymer is cellulose, found in the cell walls of plants, and used to make paper products, textiles, and cellophane.
Polymers, synthetic or natural, make up many of the materials in living organisms. This includes 1) proteins, a great nutritional value, 2) cellulose, the most plentiful of all natural polymers, and 3) nucleic acids, which are capable of being broken down to yield phosphoric acid, sugars, and a mix of organic bases (purines and pyrimidines). What does this mean? Polymers are crucial to the basic structure in vital life processes. Polymers are in our bones, our skin, our hair. They’re in our DNA. They are in the food we eat providing proteins (meat and dairy), starch (potatoes and pasta), and cellulose in green vegetables.
But where does energy come in to play? It comes from these polymer chains which contain significant amounts of energy. Any nutritionist will tell you the importance of proteins. Although they may not recommend copious amounts of starches, and definitely won’t say “eat cellulose”, you can be sure they will tell you to eat green vegetables! There is energy in these polymers! But does this apply only to natural polymers?

In a previous article we learned that crude oil is composed of hydrogens and carbons, or hydrocarbon molecules. Two characteristics of hydrocarbons are that they contain a lot of energy and they can take on many different forms. The smallest hydrocarbon is methane, a gas lighter than air. Longer chains of hydrocarbons are in liquid form. Hydrocarbon chains are versatile; however, crude oil contains hundreds of types of hydrocarbons all mixed together. They must be separated to become useful. This is where refining comes in, separating the different hydrocarbons into components or fractions in a process called fractional distillation. Next the fractions are treated for impurities, cooled, and prepared for polymerization.
As we know, hydrocarbons are made of molecules, which are made of monomers. Monomers are polymerized to become polymers. Polymers are used to make products such as gasoline, lubricating oils, kerosene, all of various grades, and jet and diesel fuels, heating oil, chemicals, and plastics. Chemistry is complex, but continues to be a source of great significance. Consider this: If a human could digest gasoline, a gallon would equate to 31,000 calories! Energy in a gallon of gasoline is equivalent to the energy in about 110 McDonalds hamburgers! (What is gasoline? – How Gasoline Works | HowStuffWorks)
Examples of Natural Polymers and Their Monomers
| Sources | Monomers – mono- (one) and -mer (part) | Polymers – poly- (many) and -mer (part) |
| Rubber tree, dandelion, chicle | Isoprene | Natural rubber (latex) |
| Living organisms | Nucleotides | DNA and RNA |
| Plants, algae | D-glucose | Cellulose |
| All vertebrates | a-keratin | Hair, wool, nails, hooves, claws |
| Arthropod exoskeletons, fish scales | N-acetylglucosamine | Chitin |
| Birds and reptiles | B-keratin | Feathers, reptile scales and shells |
| Plants | Glucose | Starch |
| Plants | Phenolic compounds | Lignin |
| Plants | Mostly D-galacturonic acid | Pectin |
| Insects, arachnids | Fibroin made mostly from alanine and glycine | Silk |

